Achieving consistent product quality is essential for the long-term success of a brewery. This goal represents a challenge for all brewers, and especially for those brewing products at multiple sites. Mastering product quality requires a solid approach, including a reliable quality control and quality assurance program, and a well-executed quality management system. This includes having reliable laboratory measurements focused on key chemical, microbiological, physical and sensory product parameters that enables one to consistently deliver high quality products that meet consumer satisfaction expectations.
Defining Product Quality
There are many definitions of quality, most of which fall into two general categories: (1) definitions that refer to “excellence or fit for purpose or use” and (2) those that refer to “defining characteristics.” According to Webster’s dictionary, quality is “an essential distinctive property, characteristic, or attribute.” Thus, a good definition for a quality beer is simply: “a beer that consistently meets the right set of specifications.” Achieving consistency requires a system comprised of people, technology, and processes that deliver the needed results time and time again. The main objectives of quality control are to enhance product quality, reduce risks, gain production efficiencies, and garner customer loyalty. The four dimensions of quality are product quality, service quality, process quality, and customer experience quality. Product quality is the most important dimension, as it refers to the tangible characteristics of the product, including its design, its features and its performance as evaluated by internal key metrics and validated by external consumer feedback. That final validation with the ultimate consumer is most important.
Managing Quality
Quality control (QC) is inherently a reactive process, which involves responding to measurements to prevent the product from being out of specification. The emphasis is on prevention rather than simply the detection of defects, achieving the so-called “right the first time.” QC focuses on testing products to uncover defects, detect emerging trends, trigger corrective action, and report to management to enable them to decide on product release questions when needed. For example, ensuring that all yeast used in production meets the viability/vitality standard helps to ensure that, upon pitching, final beer specifications for attributes such as real extract (RE) and alcohol levels are met. A decreasing trend in yeast viability can trigger corrective action on the yeast cooling system (e.g., if the yeast storage temperature is found to be gradually increasing). Quality assurance (QA) strives to ensure that there is an adequate Quality Management System (QMS) capable of delivering beer within specification at every production stage, making it a more proactive process that helps build quality across the operation. This includes systematic monitoring of raw materials, in-process, and final product key quality metrics. A solid QMS requires a strong QC.
The Added Challenge of Multi-Site Operations
Quality management becomes more complex in a multi-site brewing operation. An important decision a company must make is to determine how it will perform the QC function. Options include outsourcing QC to local laboratories, or setting up a central QC/QA laboratory at one site, or establishing QC/QA laboratories at all production sites. Each option has its pros and cons:
Outsourcing QC: Outsourcing QC to an outside laboratory negates the need for purchasing expensive equipment and hiring and training QC staff. However, terms of costs and lab performance metrics need to be negotiated periodically, including provisions for dealing with adjustments to the regular sample plan, as well as how expedited samples will be managed, the fees involved, and the turn-around time expectations for these. The laboratory services provider must demonstrate that they maintain proper equipment and utilize best practices such as instrument calibration and statistical quality control (SQC) tools to assure measurement reliability. A performance scorecard should show that they are able to perform these laboratory services in a consistent and reliable way.
Central Laboratory: Setting up one central laboratory for all brewing sites brings similar issues to using outside laboratory services, except that this option includes the purchasing of lab equipment, its maintenance, and the needed laboratory staffing. There will still be costs for expediting samples from other sites to the central testing laboratory and turn-around time expectations will need to be agreed upon. Reliability of equipment and testing procedures must be assured.
Site-Specific Laboratories: Establishing QC/QA laboratories at all sites is the most expensive and complex option. Identical equipment must be purchased for each site, and the necessary staffing hired. The analysis conducted at each site must be reproducible and comparable across all QC laboratories. This requires a fully functioning, high-level QC/QA system. While expensive, this option may best assure the quality of the final product.
Laboratory Requirements
Regardless of the chosen option, there are essential requirements for laboratory operations:
Standard Operating Procedures (SOPs): All analytical procedures should follow industry methods and guidelines, should be standardized and documented in SOPs. These SOPs should include methods for preparing samples, executing analysis, recording results, and error ranges. SOPs must be controlled documents with version and issue dates, managed by a proper document management system (DMS).
Technician Training: All analytical technicians should be trained and certified on the SOPs, with documentation of training dates maintained.
Reference Standards: Regular analysis of reference standards should be conducted, with results charted in a control chart. Variations should be noted, and the same sample analyzed by all technicians to compare results and address differences as needed.
Equipment Maintenance: All analytical equipment should be operated according to manufacturer’s instructions, calibrated, and maintained as specified. Documentation of all calibrations and maintenance should be kept in a database, available for audit. Ideally, identical equipment should be used across the laboratories.
Audit System: An audit system should ensure technicians follow SOPs, obtain proper results, and that equipment is calibrated and functioning correctly across all laboratories. Gaps should be recorded, reported, and corrective action plans put in place. Follow through on those action plans should be verified at the subsequent audit. The accuracy and precision of the measurements should be validated across the range of products that the brewery produces.
Proficiency Testing: Multiple laboratories should participate in a proficiency testing scheme, such as the Brewing Analytes Proficiency Scheme (BAPS) or the American Society of Brewing Chemists (ASBC) ring analysis, to control between-lab and within-lab errors. Internal ring analysis can also be conducted by sending the same sample to multiple labs for comparison.
Laboratory Information Management System (LIMS): A LIMS should control the flow of analysis, recording and reporting results to management, and serve as a data repository, essential for multiple laboratories.
Illustrating the Impacts of Measurement Consistency
Let’s examine two cases of brewing companies with multi-site operations which illustrate the importance of identifying and addressing deviations in laboratory measurements:
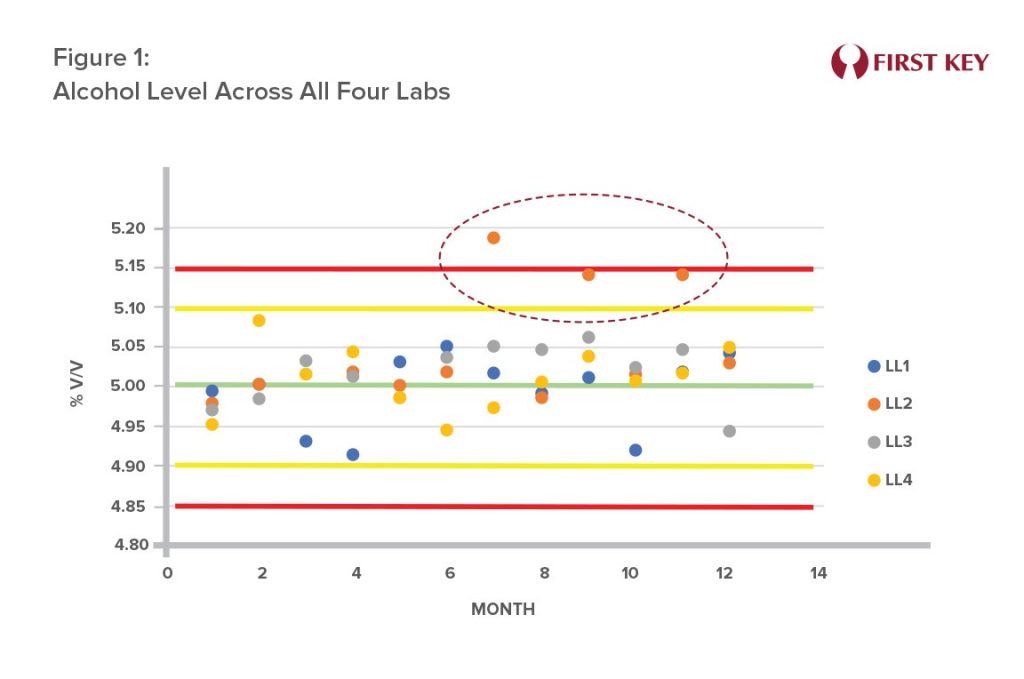
- Deviation in Alcohol Level:
A brewing operation with four sites has each site measuring key product parameters. Figure 1 shows the alcohol levels reported by each of those sites as they took part in monthly cross-checks. Lab Location 2 (LL2), showed questionable results in the second half of the year. Investigation revealed that the questionable results came from one lab technician. Retraining resolved the issue, and future analyses were within normal limits. Inaccurate alcohol levels can impact product taste and result in regulatory issues.
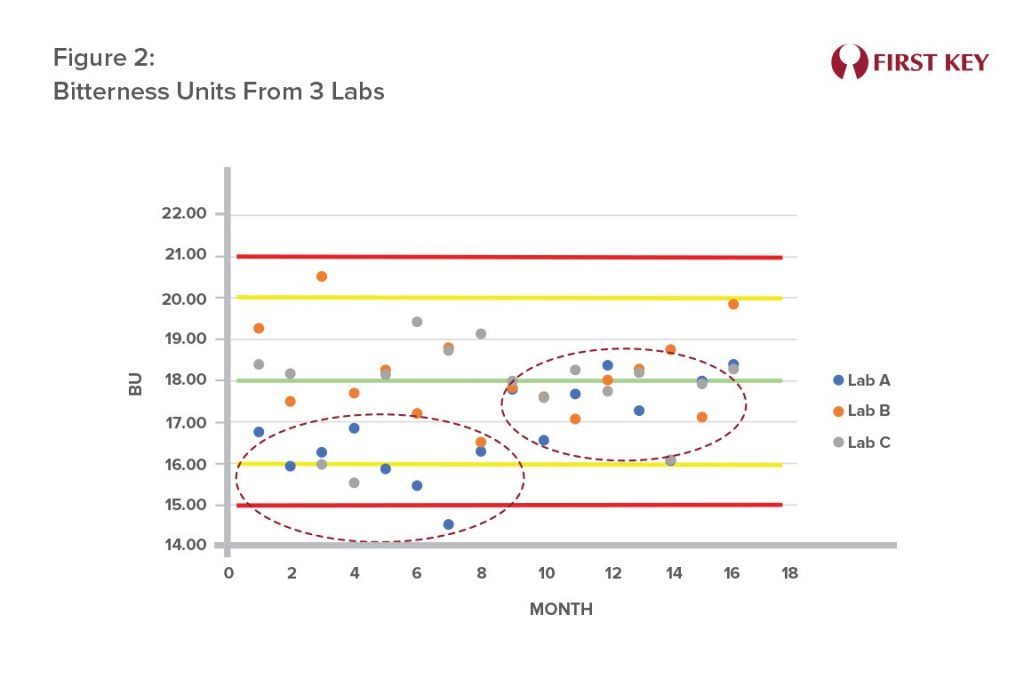
- Deviation in Bitterness Units (BU):
Figure 2 shows the case of a brewery with three laboratories where it is noticed that Lab A’s BU levels trended below the other two labs. A faulty reagent concentration was identified as the cause. Once corrected, the measured BU levels returned to normal. Inaccurate BU measurements can impact product taste and potentially increase costs due to unnecessary higher hop bills.
Summary
Achieving consistent product quality in multi-site operations requires thorough and reliable laboratory measurements of key quality parameters. Defining and implementing a comprehensive approach to assure laboratory measurements is crucial. The optimal approach for managing laboratory services varies case by case, but an excellent execution of the chosen approach will enable product quality, help assure consumer satisfaction, and with that, and improve long-term profitability.



